How to remove
Iron is one of the most common metals in the Earth’s crust, making up about 5%.
In a reducing environment, such as in deep underground waters or poorly oxygenated surface waters, iron is present in soluble form. The main forms of soluble iron in water are:
- ferrous iron – Fe2+
- ferrous sulfate – FeSO4
- ferrous bicarbonate – Fe(HCO3)2
In an oxidizing environment, on the other hand, iron is mainly present in the form of large hydroxides, which precipitate.
Iron is an essential element for the human body (e.g. for haemoglobin synthesis), and the recommended daily intake is 10 mg[1].
Iron is naturally present in water sources; generally at a maximum concentration of 0.3 mg/L, but levels may be higher in treated and supplied water, due to its use as a coagulant in aqueducts or release into water pipes due to corrosion.
Iron levels in water for human consumption should not exceed 0.2 mg/L, mainly because excess iron changes its organoleptic characteristics (colour and taste) rather than for health reasons. For this and other reasons, iron is generally removed from water when present. The bottled mineral water industry also removes iron, via oxidation by aeration or insufflation of ozone-enriched air and subsequent filtration of the hydroxides.
The main problems caused by high iron (and manganese) concentrations in water are:
- alteration of organoleptic characteristics (colour and taste),
- staining of laundry during washing (yellow due to iron and black due to manganese),
- staining and damage to tooth enamel over the long-term,
- they are detrimental to the bottling industries, laundries, papermaking, the tanning industry and to ice production,
- iron in the supply networks causes red-coloured water which may make water meter reading problematic. Excess iron also promotes the proliferation of iron-oxidizing bacteria which transform water-soluble iron compounds into insoluble oxides, causing their precipitation and accumulation in biofilm.
Multiple techniques are available to remove iron and manganese, which is often associated with iron in surface and especially in deep waters, and involve chemical or biological oxidation, or specific filtration techniques. These treatments are normally used at industrial level and not at the point of use.
Chemical oxidation
Iron can be removed via oxidation with oxygen, at atmospheric pressure if necessary, according to the following reaction:
4Fe2+ + 02 + 10H20 ® 4Fe(OH)3 + 8H+
Ferrous iron (Fe2+), the dominant form of iron in underground waters, is oxidised to ferric iron (Fe3+), forming insoluble hydroxides which can then be easily removed by precipitation or filtration.
Highly effective alternatives to oxygen include chlorine, chlorine dioxide (ClO2), and potassium permanganate (KMnO4). A process widely used in aqueducts consists of the addition of permanganate prior to filtration with sand. Iron can also be rapidly oxidised using ozone (O3), but this method is not generally used due to the high operating costs.
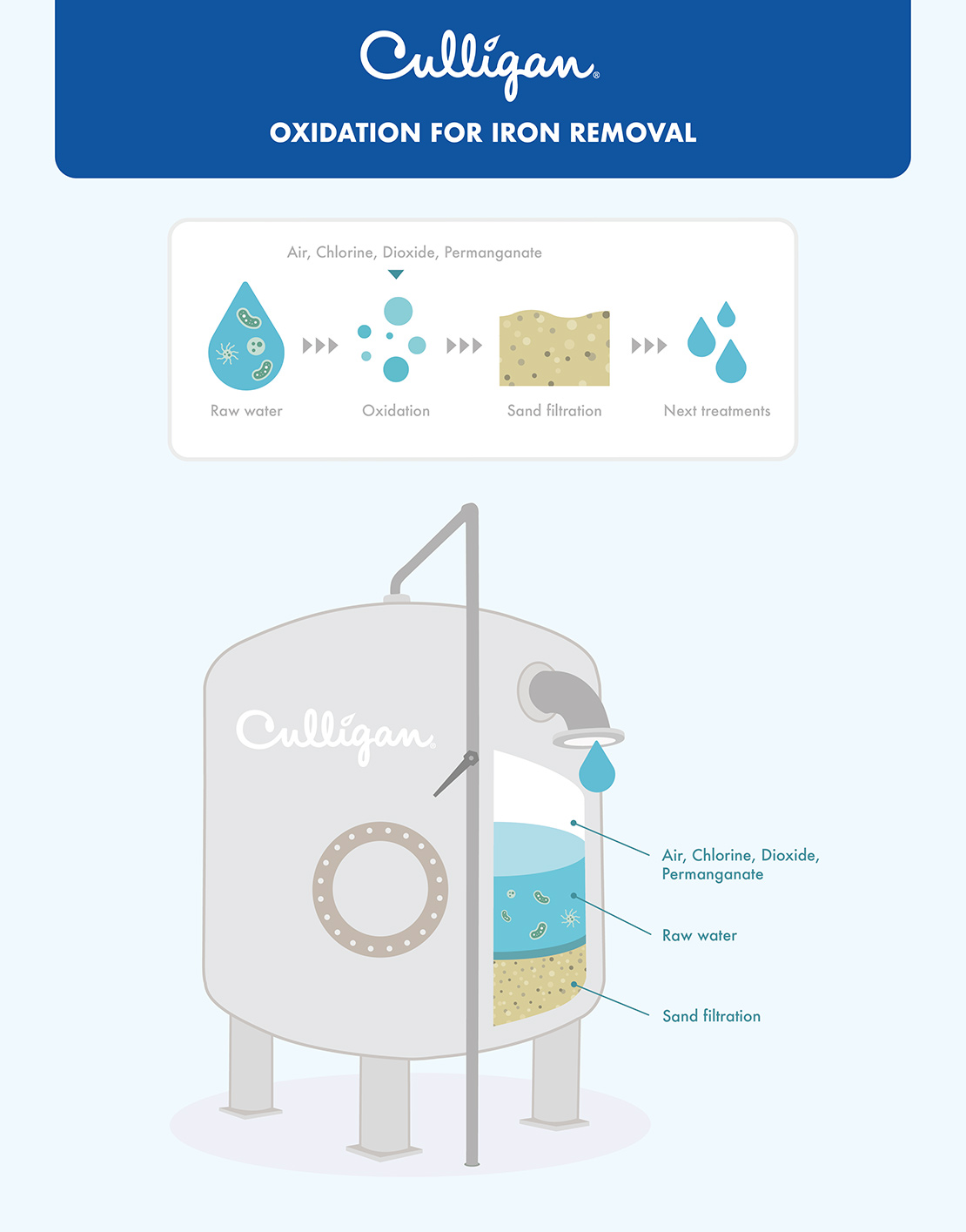
Oxidation treatment for the removal of iron
In addition to removing iron and manganese, potassium permanganate also removes tastes, smells, algae and microorganisms from water. The dose depends on the quality of the water to be treated; effective oxidation of contaminants generally requires 10mg of KMnO4 per litre of water.
Potassium permanganate is a solid which can be dissolved in water and dosed by gravity or with a dosing pump. Addition of potassium permanganate causes the water to turn violet, but this colour disappears as the compound reacts with the various pollutants during the subsequent phases of treatment.

Violet colour of water following addition of KMnO4
Filtration materials
Another effective iron removal technique consists of catalytic oxidation using granular materials containing manganese dioxide (MnO2). These materials are generally zeolites, also known as “green sand” due to the colour of the mineral, dark with greenish specks, but there are other catalytic minerals commonly used to remove iron, manganese and sulfides:
- manganese zeolite
- pyrolusite
- aluminosilicate or limestone treated with iron oxide
- activated alumina treated with iron oxide
These minerals can also remove – at least partially – other pollutants as long as these can be adsorbed on the mineral or on the precipitates formed by the mineral itself, as occurs with arsenic (already present in pentavalent form) in the treatment of waters which also contain iron.
Manganese dioxide
(MnO2) acts both as an oxygen donor and oxidation catalyst. The use of green sands typically involves a continuous dosing of permanganate, so that the mineral is used only as a catalyst, while the oxidation occurs due to the permanganate.
3Fe2+ + MnO4– + 4H+ ® MnO2 + 2Fe3+ + 2H2O
During the treatment of the water, the manganese dioxide coating the glauconite (which forms the base of the zeolite) oxidises the iron (and other ions) in the water, reducing this coating, therefore the zeolite must be regenerated using potassium permanganate, either periodically or on an ongoing basis.
Biological process
Biological treatments to remove iron and manganese use a specific type of bacteria (acidophilus), which biologically oxidise these metals, rendering them sedimentable and filtrable.
The quantity of oxidised iron grows with the increase in biomass concentration, pH and presence of ferrous iron (Fe2+), while it falls with the increase of ferric iron concentration (Fe3+).
The biooxidation of iron in biological treatments takes place via the following reaction:
2FeSO4 + 1/2O2 + H2SO4 ® Fe2(SO4)3 + H2O
The precipitates formed by biological oxidation are iron hydroxides which are more compact than those generated by chemical processes, therefore more prone to sedimentation.
With this type of treatment, the removal of iron and manganese generally occurs in two separate states. Following an initial aeration to develop the iron-oxidizing bacteria, the first biological treatment step consists of treating the water to remove the iron. This is followed by a second aeration and/or treatment to increase the pH, followed by a second biological treatment step to remove the manganese.
The main advantages of biological filtration over chemical oxidation followed by filtration are small system size, lower treatment costs and no need for chemical reagents. The disadvantages of these systems lie in the fact that the survival and proliferation of the various microbial species able to oxidise iron are strongly influenced by a variety of factors, including pH, temperature, dissolved oxygen, salinity and content of organic substances in the water to be treated.
Ion exchange
Ion-exchange resins offer another method for removing iron from water. Strong acid cation exchange resins with Na+ as a mobile ion, the conventional resins used for water softening applications, constitute one such treatment option. Since these resins are not completely selective, they act on other positive ions in the water besides iron, as well as exchanging Ca2+ and Mg2+ ions. In waters of medium-high hardness, however, calcium and magnesium ions interfere with the removal of the iron and the manganese, reducing the duration of the resin.
Despite their efficacy, these resins are not used for iron removal, since this efficacy interferes with the very technology being used.
Membrane filtration
While membranes are very efficient at removing iron and manganese, this technique is not normally used, since the oxidised forms of these metals cause fouling, even at low concentrations, drastically reducing the efficiency of the process, so much so that reverse osmosis systems (and membrane systems more generally) used to filter waters containing iron and manganese require specific pre-treatments and antiscalant treatments to reduce the risk of a sudden blockage of the membranes.
In the same way as for ion-exchange resins, membranes are not used for iron removal despite their efficacy, since this efficacy renders their use impractical.
[1] Recommended daily intake for the Italian population, 2012 (LARN Limiti di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana – Edizione 2012)

 Uffici e ristoranti
Uffici e ristoranti Per la casa
Per la casa Piscine
Piscine




